as some of you may know, i have basically spent the last year trying to purify 1-3 proteins so they can be identified. this is the last lingering torment of my PhD. to date, i have tried 3 completely different methodologies and something like 20 combinations of reagents, incubation conditions and antibody preps each. early in the fall i got one methodology to yield one of the bands, but that particular technique yielded a huge overweighting of immunoglobulin. this became my back-up, my Plan B.
when all other options were exhausted, i conceded defeat and decided to harvest the one band from Plan B. my world was completely rocked when i discovered that the protein was not present in great enough density to visualize using the less sensitive stain necessary for protein isolation. efforts to concentrate the solution further actually caused the band to disappear.
these results left me teetering in shock, wondering whether perhaps i am not PhD material.
with no other ideas and nowhere to turn, i decided to call the woman to whom i would be submitting a successful sample to see if perhaps she had any ideas. Jessica is fabulous and, after validating that this is hard to do and that many people don't succeed, came up with several great ideas. since one involved learning a fourth methodology entirely and one might compromise the sample quality, i decided to start with the easy option. i reduced my antibody concentration to 3% of the original recommendations. (note: this was not a scientific level of decrease but instead reflected the most i could decrease the antibody concentration without having to make a separate stock dilution. basically, i was disheartened and lazy.]
of course the concentration of protein in my sample was far too minute to show up using the cursed (pronounced cur-sed in the style of movie villains) Coomassie stain.
so i spent three days running the column over and over and over and over and over and over and over and over and over again...
...on the same lysate sample since i knew there was plenty of protein in the sample, just a persnickety recalcitrance on the part of the antibody to bind it. yesterday afternoon, knowing i was headed to a committee meeting today and uncertain of how hard to campaign for a failed experiment chapter in my dissertation, i decided to run what i had. before i started, i instructed Jen (my patient, supportive and awesome labmate/friend) to remind me i expected it not to work when it ended up failing. by the time Jen left for the night, i had 20 minutes destaining to go and it was a pretty obvious failure, so she consoled me and i took it well.
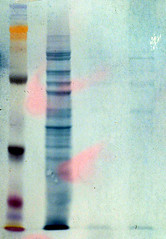
twenty minutes later, this is what i slid out onto the glass in preparation to through it in the trash.
and there it was. my sweet, sexy, playful, perfect friend - the ~40 kD band.
you are looking at the blob of slimy goo and thinking, 'She is truly unhinged. There is nothing there.'
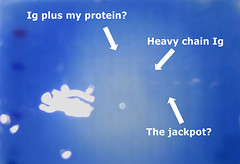
but it was obvious when held at an angle with the light shining through it. i dragged my advisor down to witness the glory of the band before i cut it out to save for Jessica. unfortunately, to avoid potential keratin contamination (read failed experiment), i could not risk taking it to the image capture system for a photo. so i did the best i could with my camera through the plexiglass hood and without a mount.
if you squint your eyes up till you feel a headache coming on and use the membrane at the top of the post as a guide, you can almost believe what you are seeing on this photo, annotated for your viewing pleasure...
i could cry. i could laugh. i could laugh until i cry.
Jen and i are going for margaritas at the Rio on Saturday to to celebrate. you are welcome to join us.



1 comment:
So I am patient? Always nice to hear that I am able to confuse others with my wit and smiling, while secretly going insane and banging my head against the bathroom stall. Really the bathroom seems to be the perfect solace for a bad day...cool, somewhat disinfected and a nice comfortable chair is all you really need...
Post a Comment